Autoimmune diseases have a way of reshaping a person’s life slowly and silently. One morning it’s stiff joints. Another day it’s unexplained fatigue. Eventually, a pattern emerges: the body seems to be reacting to its own tissues as though they were foreign invaders. For many people exploring nutritional strategies to support their health, lectins, those carbohydrate-binding proteins found in beans, grains, legumes, and nightshades, have become part of the broader conversation about why the immune system misfires and how diet might contribute.
But this conversation is rarely simple. Lectins occupy a strange space somewhere between nutritional villain and misunderstood plant defense mechanism. The truth resides in a nuanced middle ground: there is promising evidence, intriguing theories, and important limitations that must be acknowledged. Understanding where these ideas stand today requires walking through the science with patience rather than jumping to conclusions.
Why Lectins Entered the Autoimmunity Debate
Lectins became a point of interest long before autoimmune diseases became common terminology in mainstream health discussions. In the early and mid-20th century, researchers studying plant proteins discovered that lectins could bind to the intestinal lining and sometimes provoke cell changes or immune reactions in laboratory settings. Scientists were fascinated by these proteins because they weren’t like ordinary food molecules that break down during digestion, they were resistant, durable, and capable of interacting directly with human tissue.
As research expanded, some studies suggested lectins might alter gut barrier integrity, influence immune signaling, or mimic molecules that the body mistakes for self-tissue, a process known in autoimmune theory as molecular mimicry. These findings drew attention from people who were already noticing that certain foods triggered flares, gut discomfort, or fatigue.
However, lectins are not inherently dangerous. Many are rendered harmless through cooking, pressure cooking, fermenting, or sprouting. And most people consume them every day without developing autoimmune disease. The question, then, is not whether lectins cause autoimmunity universally, but whether they may act as contributing factors in susceptible individuals.
The Gut Barrier as a Central Player
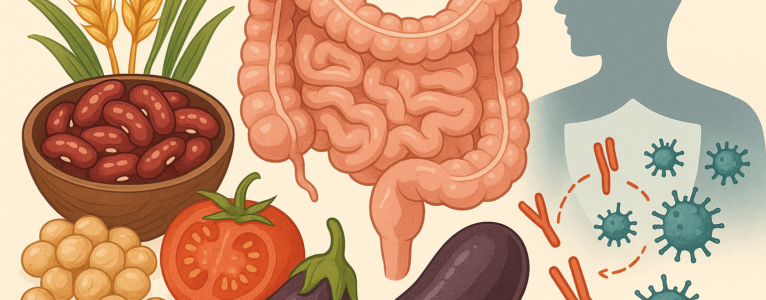
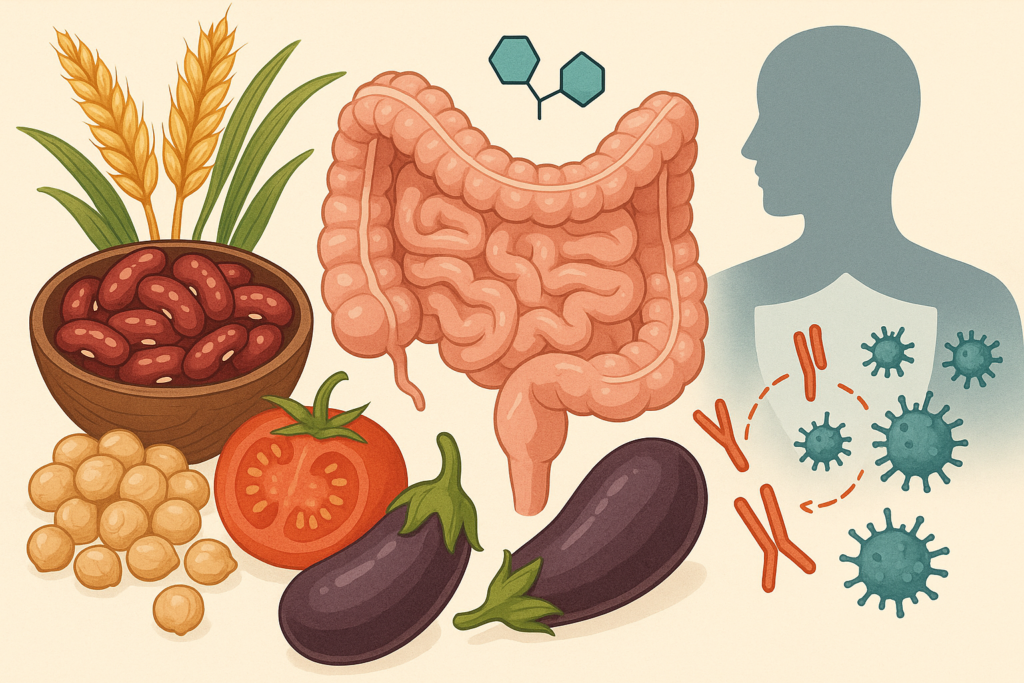
To understand how lectins might influence autoimmunity, it helps to first look at the gut barrier, a system that is both delicate and incredibly strong. Picture it as a tightly regulated security checkpoint: nutrients are ushered through with care, while unwanted particles are kept out.
Autoimmune researchers have spent decades studying the ways this barrier can weaken or become overly permeable, allowing larger molecules to slip past the tight junctions and interact with the immune system in ways they shouldn’t. This state, often described as “increased intestinal permeability,” has been observed in various autoimmune conditions, from celiac disease to rheumatoid arthritis.
Some animal studies and cell models suggest that certain lectins can bind to intestinal cells and may increase permeability under the right conditions. For example, raw or undercooked beans contain lectins that are known to irritate the gut. But these conditions do not reflect typical human cooking practices, nor do they guarantee that lectins behave the same way in every person.
Still, in individuals with genetic predispositions, existing inflammation, or immune dysregulation, even small irritants can contribute to a cascade of immune confusion. This is where lectins become part of the broader story, not as the sole villain, but as one variable influencing an already complex system.
Molecular Mimicry: A Compelling but Unproven Idea
One of the most dramatic theories connecting lectins to autoimmunity is molecular mimicry. This idea suggests that certain food proteins resemble parts of human tissue closely enough that once the immune system reacts to the food protein, it may accidentally begin reacting to a similar-looking protein in the body.
In the case of lectins, the theory proposes that lectins may bind to or resemble carbohydrate structures involved in immune signaling. If the immune system interprets these bindings incorrectly, the response could escalate over time.
This concept isn’t exclusive to lectins, the same theory has been applied to gluten, dairy proteins, viruses, and even bacteria. Molecular mimicry has been observed in some well-documented autoimmune cases, such as rheumatic fever triggered by a Streptococcus infection. But when it comes to lectins specifically, the evidence is much more limited.
The difficulty is that autoimmune diseases are not caused by a single trigger. They require a perfect storm: genes, environment, immune priming, stress, infections, gut health, and a host of other influences that accumulate over months or years. Lectins may contribute to this environment for certain individuals, but proving that they initiate the autoimmune process remains elusive.
What Human Research Actually Shows
When the public hears about lectins and autoimmunity, much of the discussion stems from animal and laboratory studies, some of which use doses or conditions not comparable to real human diets. Actual human research on lectins is sparse, and when it does exist, it often reveals a more restrained message.
For instance, studies on cooked legumes, one of the richest lectin sources, consistently show health benefits for most populations: improved weight management, better blood sugar control, beneficial gut microbiota shifts, and reduced markers of inflammation. These outcomes do not suggest that lectins universally cause immune dysfunction; if anything, they underscore the importance of studying context, not just isolated molecules.
A few small-scale trials have explored whether reducing lectins might alleviate symptoms in autoimmune-like syndromes, but these studies typically combine multiple dietary changes at once. When participants experience improvement, it becomes difficult to attribute the change specifically to lectins rather than overall reduced inflammatory load, improved nutrient density, elimination of processed foods, or changes in gut bacteria.
This is the limitation of diet research more broadly: humans do not eat single molecules, and the interplay between food, metabolism, and immune function is complicated enough that isolating one protein class is extremely challenging.
Autoimmune Conditions Where Lectins Are More Heavily Suspected
Although the evidence is mixed, there are certain autoimmune or immune-related conditions where lectins receive more scrutiny:
Celiac Disease
Celiac disease involves a highly specific immune response to gluten, not lectins per se, but researchers have noted that lectins from wheat germ (wheat germ agglutinin) may amplify gut irritation in susceptible individuals. The lectin is not the cause of celiac disease but might worsen the inflammatory environment.
Rheumatoid Arthritis
Some older studies reported that certain lectins could influence collagen-binding or joint-related immune responses. However, these findings have not been consistently replicated in modern studies.
Inflammatory Bowel Disease
Animal and tissue studies suggest that lectins may aggravate inflammation in the gut lining under certain conditions. Yet human studies are inconclusive, and many patients tolerate cooked lectin-containing foods well.
Hashimoto’s Thyroiditis
There is no direct evidence linking lectins specifically to thyroid autoimmunity, though many individuals report reduced symptoms when removing inflammatory trigger foods more broadly, some of which happen to be high in lectins.
These areas of suspicion should not be confused with proven causation. Instead, they serve as starting points for future research and reminders that individual sensitivity can exist even in the absence of universal patterns.
When Reducing Lectins Helps: The Individual Puzzle
People exploring low-lectin eating often don’t arrive there out of curiosity. They arrive because something in their body is asking for change. Chronic bloating, joint pain, brain fog, skin issues, or unpredictable energy levels often lead them to experiment with diet.
Many discover that lowering lectins brings a surprising degree of relief. But the reasons behind that relief are varied:
- They may have reduced overall inflammation by removing large categories of foods.
- They may have eliminated highly processed items indirectly.
- Their gut microbiome might shift due to a change in dietary fiber types.
- They may have removed specific trigger foods unique to their physiology.
If lectin reduction helps someone feel better, that experience is valid even if the mechanism isn’t fully understood yet. Autoimmune disease management often requires this kind of experimentation, because no single dietary pattern fits everyone.
However, some people eliminate lectins unnecessarily and end up with overly restrictive diets that are difficult to sustain. The key is understanding that dietary tools should be used with nuance… not fear.
Where the Scientific Gaps Remain
As fascinating as lectin research is, it still contains more open questions than answers. Current limitations include:
1. Lack of large-scale human studies – Research tends to rely on small sample sizes or mechanistic theories that don’t reflect real-world conditions.
2. Difficulty isolating lectins as the sole variable – Dietary research is messy because foods contain complex mixtures of nutrients and compounds that all interact in the digestive system.
3. Variability in lectin sensitivity – Some people tolerate lectins easily; others do not. The reasons remain unclear.
4. Differences in food preparation – Cooking destroys or reduces lectins significantly, yet many studies use raw lectins in test conditions.
5. Individual microbiome diversity – Gut bacteria heavily influence how lectins behave in the digestive system. Two people eating the same lectin-rich meal may experience completely different biological outcomes.
Until these gaps are filled, lectins remain an intriguing but unsettled component of the autoimmune conversation.
A Balanced Perspective Moving Forward
For many individuals, exploring the relationship between lectins and autoimmunity becomes part of a larger quest to understand their body’s needs. Food turns into both medicine and messenger, signaling patterns that medical tests sometimes struggle to reveal. Removing lectins for a period, then reintroducing them with care, can help people identify whether these proteins play a meaningful role in their symptom patterns.
But lectins should not be viewed in isolation or portrayed as universally harmful. They exist within a complex dietary landscape where cooking methods, gut health, genetics, and immune variables all shape the body’s response. For some people, lectins may be irritants. For others, they may provide beneficial fibers that support metabolic and digestive health.
Autoimmunity is too multifaceted for simple explanations, and the path forward requires humility on both scientific and personal levels. Researchers must continue exploring without overstating conclusions, and individuals must listen to their bodies without assuming that what works for one person works for everyone.
In the end, the discussion about lectins and autoimmunity is not about finding a single culprit. It’s about understanding biological nuance, respecting individual variation, and using dietary tools thoughtfully. With continued research and personal experimentation, the role of lectins will become clearer, and people navigating autoimmune challenges will have more informed options for supporting their long-term well-being.